Diseases caused by protozoans are common problems in aquarium fishes, and several have been observed in zebrafish. Ichthyophthirius multifiliis (the cause of “Ich” or white spot disease“) and Piscinoodinium pillulare (the cause of freshwater velvet disease) are very common in warm water captive fishes. The latter is recognized as a cause of disease in zebrafish held in research facilities. Microsporidia are generally more host specific and one of these, Pseudoloma neurophilia, is very common in zebrafish from research facilities and pet stores (de Kinkelin 1980; Matthews et al. 2001).
Piscinoodinium pillulare, the cause of velvet disease, is a yellowish, parasitic dinoflagellate in which one stage infects the skin and gills of fish. The parasite can multiply very rapidly in aquaria and will rapidly kill fish. This is a common pathogen of ornamental fishes. The dinospore stage infects fish, and transforms into a trophont stage that attaches to the surface, especially gills, and apparently feeds on the host’s epithelium. After several days, this stage leaves the host and forms a tomont. This off-host stage undergoes several divisions, resulting in 256 infective motile dinospores which go on to infect other fish. The life cycle is completed in about 2 wk under optimal conditions (ca 23-26 oC).
Heavily infected fish exhibit lethargy, may hang near the surface of the water column, and exhibit labored breathing. Fish may exhibit a greyish to rusty color sheen on the surface.
Wet mount examination of the skin or gills reveals numerous, oval, opaque non-motile trophonts (about 9-12 X 40-90 µm). In histological sections, Piscinoodinium appears as oval organisms on the gill and skin surface, with numerous cytoplasmic (often refractile) granules and a large nucleus. Infections of the gills are associated with prominent epithelial hyperplasia. Similarly, skin infections result in severe hyperplasia and erosion of the epithelium.
The infection is diagnosed by observation of the trophonts in either wet mounts or histological sections. For the former, Piscinoodinium is distinguished from many other surface-infecting protists by it’s lack of motility.
Control and Treatment. We do not have personal experience treating Piscinoodinium infections with zebrafish. Noga (1996) recommended prolonged immersion in salt at 1 teaspoon/5 gal. Very heavy, life-threatening infections may be treated with a quick (1-3 min) bath in full strength sea water (35 ppt). As with Ich, the presence of off-host developmental stages should be considered when implementing a treatment regime. Therefore, it is best to remove all the fish, treat them in a new aquarium, and disinfect the original tank.
Pseudoloma neurophilia
Neural microsporidiosis in zebrafish was first reported in 1980 in France (de Kinkelin 1980). The parasite has now been identified in zebrafish at many research and commercial facilities, and was assigned to a new genus and species, Pseudoloma neurophilia by Matthews et al. (2001). The infection is linked to severe emaciation (often referred to as ‘skinny disease’), but its precise role in every case (primary cause or secondary opportunist) has yet to be resolved. In a survey of a population with a known high prevalence of infection, 97% of the skinny fish and 30% of the normal fish were infected. This demonstrates that, as with many parasites, the infection may be also found routinely in normal, healthy appearing fish. Conversely, there are several other causes of emaciation in zebrafish. Handling and crowding stress causes increased severity of the infection, along with reduced growth and fecundity (Ramsay et al 2009).
Microsporidia are obligate intracellular fungus-like parasites with a complicated life cycle. The life cycle concludes with the production of an infectious and resistant spore, which is the only stage of the parasite that can live outside of the host cell. With most microsporidia, transmission occurs via the ingestion of the infective spore stage, and we have found the infection in exposed adult fish as soon as 4 wk after feeding infected tissues. Fry fish are very susceptible, and may show a more acute form of the disease, with high mortality about 1 wk post exposure (Ferguson et al. 2007). While the parasite is easily transmitted by feeding on infected carcasses, we have found that it is also transmissible when fish are kept physically separate, but in the same water. We presume this is by infected egg material, etc. being released from infected females, as zebrafish frequently spawn under natural aquarium conditions.
There have also been reports of vertical transmission in other microsporidia of both vertebrates and invertebrates (Bandi et al. 2001; Dunn et al. 2001; Phelps et al. 2008). We detect the spores of Pseudoloma in the ovaries and sometimes within immature and degenerate eggs of zebrafish, and the role of vertical transmission or pseudovertical transmission (transmission to progeny with the pathogen outside of the egg) of Pseudoloma is currently under investigation.
Emaciation and spinal curvature, such as scoliosis, are common in infected fish. However, emaciation and spinal curvature are nonspecific signs with numerous etiologies. Furthermore, clinically normal zebrafish may also harbor heavy P. neurophilia infections. Therefore, diagnosis of microsporidiosis should not be based on clinical signs alone.
The primary site of infection is the central nervous system (spinal cord and hindbrain) and nerve roots. The infection often extends to the skeletal muscle, where it causes severe, chronic, multifocal inflammation. It also infects the ovaries and eggs, and occasionally other organs, such as the kidney (Kent and Bishop-Stewart 2003).
The infection can be detected in wet mounts of the central nervous system that have been carefully dissected from infected fish. Because this is tedious and laborious, histology is the routine method by which the infection is observed. Spores are ovoid to pyriform, with a prominent posterior vacuole, and average 5.4 x 2.7 um. The microsporidium produces xenomas within the spinal cord and hindbrain of fish, and xenomas contain sporophorous vesicles with up to 16 spores. Sporoblasts and presporoblast stages (probably sporonts) are found rarely in small aggregates dispersed randomly throughout xenomas. Fungi-Fluor (Polysciences, Warrington, PA) fluorescent stain binds nonspecifically to beta-linked polysaccharides found in cells containing chitin. As chitin occurs in spore walls of microsporidia, this stain is excellent for demonstrating spores in either tissue smears or histological sections. Weber et al. (1999) describes a related stain (Calcofluor) and other staining techniques for the identification of microsporidia. Acid fast and Gram stains are also very useful for demonstrating microsporidian spores, and we have found that the Accustain® Gram stain (Sigma-Aldrich) on sections is excellent for this purpose. Whipps et al. (2006) developed a PCR test for P. neurophilia. Recent work at both ZIRC and OSU has resulted in the development of even more sensitive PCR tests, and we are currently developing a non-lethal test based on PCR of water and eggs. We were able to establish a new facility that, to date, has remained free of the infection by pre-screening all brood fish by PCR.
Ultraviolet light (UV) is used to control pathogens in zebrafish systems. UV sterilization has been successfully employed to kill viruses, bacteria, and protozoa in water before coming in contact with fishes, in both flow through and recirculating systems. Most systems use UV sterilizers following biological filtration. Other microsporidian species have been shown to be very susceptible to UV treatment, and our observations at ZIRC strongly indicate that UV at 30-50,000 µWsec/cm2 kills the parasite and thus prevents it transmission in a recirculating system. Fumagillin has been widely used as an oral treatment for fish microsporidiosis, usually with good success (see review by Shaw and Kent 1999). Fumagillin was first developed for treating Nosema apis infections in honey bees. Kano et al. (1982) reported that fumagillin was effective against the microsporidium H. anguillarum in eels (Anguilla japonica). Since this first report on treating microsporidiosis in fish with fumagillin, the drug has been used to treat N. salmonis infections in Chinook salmon (Hedrick et al. 1991) and Loma salmonae infections in Chinook salmon (Kent and Dawe 1994). Our initial investigations with this drug were not successful for controlling or reducing the infection in zebrafish, and we plan to repeat the study using higher doses as we found no side effects with the drug.
Microsporidian spores may be resistant to disinfectants (Shaw et al. 1999), and Ferguson et al. (2007) found that chlorine levels routinely used by zebrafish facilities (i.e., 25 or 50 ppm for 10 min) was not effective for killing spores. Moreover, we have observed eggs filled with spores of Pseudoloma in histologic sections of ovaries (see figures). The spores within intact eggs could be protected from chlorine, even if this concentration is effective for killing spores.
Based on our understanding of the parasite at this time, the following practices would likely reduce the spread and impact of the infection 1) ensure that water in recirculating systems is adequately treated with UV sterilization, 2) with existing infections, remove and euthanize all emaciated and moribund fish as soon as possible to prevent cannibalism and further transmission, 3) consider screening of brood fish, particularly females, for the parasite to reduce the risk of transferring the infection into a facility with their progeny, 4) rear potentially infected fish in optimum conditions and reduce stress to minimize the impact of the infection, and 5) minimize potential for cross-contamination of stocks by assigning dedicated populations of wild-type fish for out crossing to each mutant stock. After breeding, these wild-type fish should not be returned to a common stock tank nor be crossed to any other mutant stock.
Recently we have diagnosed another microsporidium, Pleistophora hyphessobryconis, in a few zebrafish research facilities. This parasite, the cause of neon tetra disease, infects a wide variety of aquarium fishes, including tetras, barbs and goldfish (Lom and Dyková 1992). The infection results in massive numbers of developing parasites and spores throughout the skeletal muscle (see figure below), and spores are found in other organs within macrophages. This parasite certainly should be avoided in zebrafish research facilities, and thus we recommend extreme caution using zebrafish that have come in contact with other aquarium fishes, particularly tetras. Diagnosis, control and treatment are similar as those for Pseudoloma, but a PCR test for Pleistophora hyphessobryconis in not presently available.
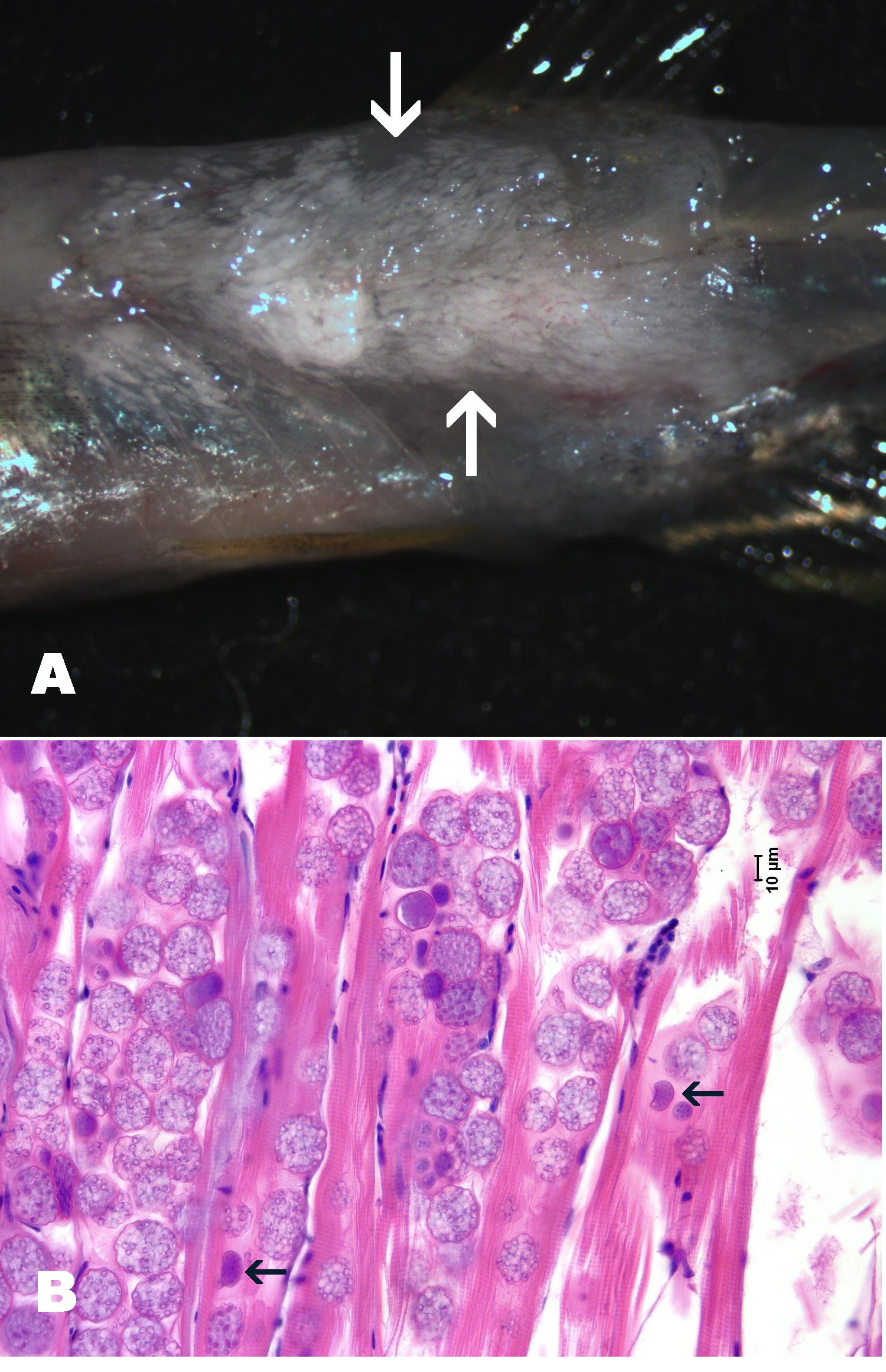 **Click for high resolution image** | Massive infection by Pleistophora hyphessobryconis in zebrafish. A. Infected zebrafish with skin removed showing whitish opaque areas in muscle due to the infection. B. Numerous sporophocysts with mature spores infect the muscle fibers. Arrow = dark staining sporonts with immature, developing spores. |
Ich, caused by the ciliate Ichthyophthirius multifiliis, is probably the most important disease of freshwater aquarium fishes. Fortunately, it has not been recognized as an important problem in zebrafish research facilities, but as essentially all aquarium fish are susceptible to the infection it warrants review here. Some similarities in the life cycle are shared by the Ich parasite and Piscinoodinium. Both have off-host encysted stages in which multiplication of organisms occurs, followed by free-swimming stages infecting fish. In contrast, Ich penetrates the epithelium, and thus makes it more difficult to eradicate with external baths or dips. Noga (1996) and Dickerson and Dawe (1995) provide reviews on Ich.
Clinical Disease and Gross Pathology. Fish with heavy infections exhibit excessive mucus production, labored breathing, and lethargy. A hallmark of the disease are white, raised nodules on the skin, hence another common name for the disease is “white spot”.
Wet mount preparations of the skin and gills reveals motile ciliates varying in size, from the initial infective stage (theront) of about 25 in length to the stage embedded under the epithelium reaching up to 1 mm in diameter. Histological sections reveal the parasite under the epithelium associated with severe epithelial hyperplasia. A large, horseshoe-shape macronucleus may be visible in wet mount or tissue sections.
Observation of active ciliates of variable size in wet mounts of the skin or gills is a good presumptive diagnosis. Definitive diagnosis is achieved by observing the distinctive macronucleus and observing parasites under the epithelium of the gills or skin.
Control and Treatment. Extensive literature is available on control strategies for Ich, and the most common treatment is an external bath with formalin. Usually fish are treated with formalin at about 1:4-5,000 formalin for 1 hour. Each fish species responds differently to formalin, and thus a few fish should be tested before applying the treatment to large numbers. The treatment must be carried-out diligently as the stages under the skin are somewhat protected from external baths. In other words, multiple treatments may be required. Furthermore, the entire system should be treated to destroy the off-host stages of the parasite, which may be detrimental to biological filters.