Metazoan parasites (helminths and arthropods) are important causes of disease in captive fishes, including warm water species. Because zebrafish colonies usually use dechlorinated city water and are maintained in closed systems, metazoan parasites (particularly those requiring intermediate hosts) are not numerous in these facilities. Capillarid nematodes are one of the few metazoans that have been recognized as a problem in zebrafish facilities (Pack et al. 1995; Kent et al. 2002).
The species infecting zebrafish was first identified as Pseudocapillaria tomentosa by Dr. R. Overstreet, University of Southern Mississippi (pers. comm.), and the specimens that we have evaluated from zebrafish are consistent with this identification. Capillarids infect all classes of vertebrates, and are often pathogenic due to their invasive nature (Moravec 1987; Moravec et al. 1987a ). Many species have been described from fishes, including aquarium species (Williams and Jones 1994).
Pseudocapillaria tomentosa (junior synonym P. brevispicula) has a broad host specificity, infecting some 25 fishes in the family Cyprinidae and members of other orders such as Aguilliformes (eels), Gadiformes (cod fishes), Salmoniformes (salmon) and Siluriformes (catfishes) (Moravec 1987). Pseudocapillaria tomentosa was associated with mortality in captive tiger barbs (Puntius tetrazona) (Moravec et al. 1984) and related parasites cause disease in other aquarium fishes. Capillaria pterophylii has been recognized for many years as a common pathogen of captive angelfish and discus fish (Richenbach-Klinke 1952) and Capillostrogyloides ancistri is highly pathogenic to the bushymouth catfish Ancistrus dolichopterus (Moravec et al. 1987b). Lomankin and Trofimeko (1982) showed that oligochaetes (e.g., Tubifex tubifex) can serve as paratenic hosts for P. tomentosa in laboratory transmission studies. In the same study, they demonstrated that direct transmission in the absence of worms is also a route of infections. Recently, we confirmed that the parasite can be transferred from fish to fish in the absence of oligochaete worms, which demonstrates that the parasite can proliferate in zebrafish research facilities.
Heavily infected fish are often dark, emaciated, and lethargic. Necropsy may reveal liver enlargement and anemia.
Females of P. tomentosa are long (7-12 mm) and thin, and gravid worms are replete with the distinctive eggs that are visualized in wet mount preparations of the gut. Males are smaller, about 4- 7 mm in length. Histological sections reveal the worms within the gut wall and, at times, are associated with a severe cellutitis of the infected region The tissue reaction can be severe, diffuse and may extend through the entire visceral cavity. The infection also seems to predispose fish to intestinal neoplasms (Kent et al. 2002) – see Neoplasia.
Identification of capillarid nematodes is most easily accomplished by the presence of unique eggs, which are oval and contain distinctive bipolar plugs. Precise identification to the species level requires careful examination of the male sexual organs, which are rather diminutive in Pseudocapillaria spp. Females of P. tomentosa are about 7-12 mm, males about 4-7 mm. To date, the only nematode infection that we have identified in zebrafish from research facilities is P. tomentosa.
Oligochaete worms may be a source of the infection, and thus should be avoided as food, particularly if their source is unknown. As direct transmission occurs between fish, the infection can spread within a population if not controlled. If fish are not highly valuable, the most appropriate choice would be to eliminate the infected population. At present, the infection is not wide spread in research facilities, perhaps due to bleaching of eggs and quarantine procedures.
Ivermectin is a very effective antihelminthic for many nematode and arthropod infections in terrestrial animals, and has been employed for treating nematode infections in fishes (Heckman 1985). Therefore, ivermectin should be considered as a possible treatment for P. tomentosa in zebrafish. However, this drug has not been tested with zebrafish, and there appears to be a great variability is the tolerance of the drug between even closely related fish species – i.e., many fish species are highly susceptible to toxic side effects of ivermectin at doses used in mammals (Johnson et al. 1993: Kilmartin et al. 1997), presumably because of a comparatively reduced blood/brain barrier causing neurotoxicity. This drug has also been associated with respiratory problems in fish (Toovey et al. 1999).
Oral or bath treatments with levamisole and fenbendazole have also been employed to treat nematode infections in fish (Noga 1996), but Hoffman (1982) found that levamisole was not effective for treating capillarid infections in golden shiners. Brood stock zebrafish treated with levamisole have become sterile (D. Weaver, Scientific Hatcheries, Huntington Beach, California, pers. comm.). Pack et al. (1995) reported that a mixture of trichlorofon and mebendazole in the form of Fluke-Tabs (Aquarium Products, Glen Burnie, MD) added to water eliminated the infection. Treated fish gained weight and when examined later the infection was not observed. Dr. Weaver has reported success with treating P. tomentosa infections using pyrantel and garlic powder incorporated in the feed in a production situation.
Myxozoan parasites (phylum Myxozoa, class Myxosporea) are common parasites of cold-blooded vertebrates, particularly fishes (Kent et al. 2003). Traditionally the Myxozoa have been classified with the Protozoa. However, about 15 years ago analysis of small subunit ribosomal DNA revealed that the Myxozoa rooted within the kingdom Animalia. Two viewpoints have predominated in these discussions. One suggests that myxozoans are most closely related to the Cnidaria (diploblasts), due to the morphological and developmental similarities of their polar capsules and nematocysts respectively. These observations and phylogenetic analysis in support of them were first presented in a key publication by Siddall et al. (1995). Other analyses support phylogenetic proximity to the bilaterian animals (triploblasts) (Zrzavy et al. 2003).
There are over 2,200 described species of myxozoans, and most are relatively non-pathogenic (Lom and Dyková 2006). Those that that infect lumina of organs are called coelozoic species. These are commonly found in the gall bladder, bile ducts, or urinary tract, and they often cause little tissue damage. Histozooic species (those occurring deep within tissues) usually form small, confined white cysts with little associated tissue damage. However, when these cysts are numerous in vital organs, such as the gills or heart, they can cause disease.
The life cycle of myxozoans is complicated (see figure below). They contain several vegetative stages (trophozoites) and development in the fish culminates in the formation of multicellular spores, called myxospores. Spore morphology is the primary criterion used for identification of myxozoans. Development in an aquatic oligochaete or polychaete is required to complete the life cycle (Kent et al. 2003). The forms found in oligochaetes were originally considered to be different parasites, which were assigned to the class Actinosporea. Because these stages are not separate taxa from myxozoans found in fishes, the taxonomy of the phylum Myxozoa has been revised (Kent et al. 1994).
Whereas serious myxozoan diseases are found in wild or cultured fish with access to open waters, they are less often seen in laboratory systems. This is likely because of the requirement of the parasite to pass through an annelid worm to infect another fish. We have, however, detected a myxozoan, belonging to the genus Myxidium or Zschokkella in zebrafish from several research facilities.
To date we have not associated clinical disease with the infection.
Histological sections of infected fish reveal the parasite in the common mesonephric ducts and occasionally in the lumen of kidney tubules. Infections may be heavy, but are seldom associated with significant histological changes. The organism seen in zebrafish is comprised of a multicelluar trophozoite (plasmodium) in which careful examination reveals myxospores inside. The hallmark identifying character of myxospores is the presence of polar capsules, which can be better visualized with special stains such as methylene blue and Giemsa. The genera Myxidium or Zschokkella have members with polar capsules located on the opposite end of the spores.
The infection is diagnosed by observation of the parasite in histological sections. Wet mounts of tissues are often used to visualize myxospores.
Infections in a zebrafish facility indicate that the oligochaete host is present in the water system (e.g., tanks or filters). Indeed, we have seen reports of small oligochaetes (e.g., Stylaria spp.) in zebrafish system. In addition, oligochaetes used to feed fish (e.g., black worms, red worms) may be sources of myxozoan infections to fishes held in aquaria (Hallett et al. 2005). Fumagillin and a few other drugs show variable efficacy for treating myxozoan infections. Treatment of the zebrafish myxozoan would probably not be warranted as it is not very pathogenic.
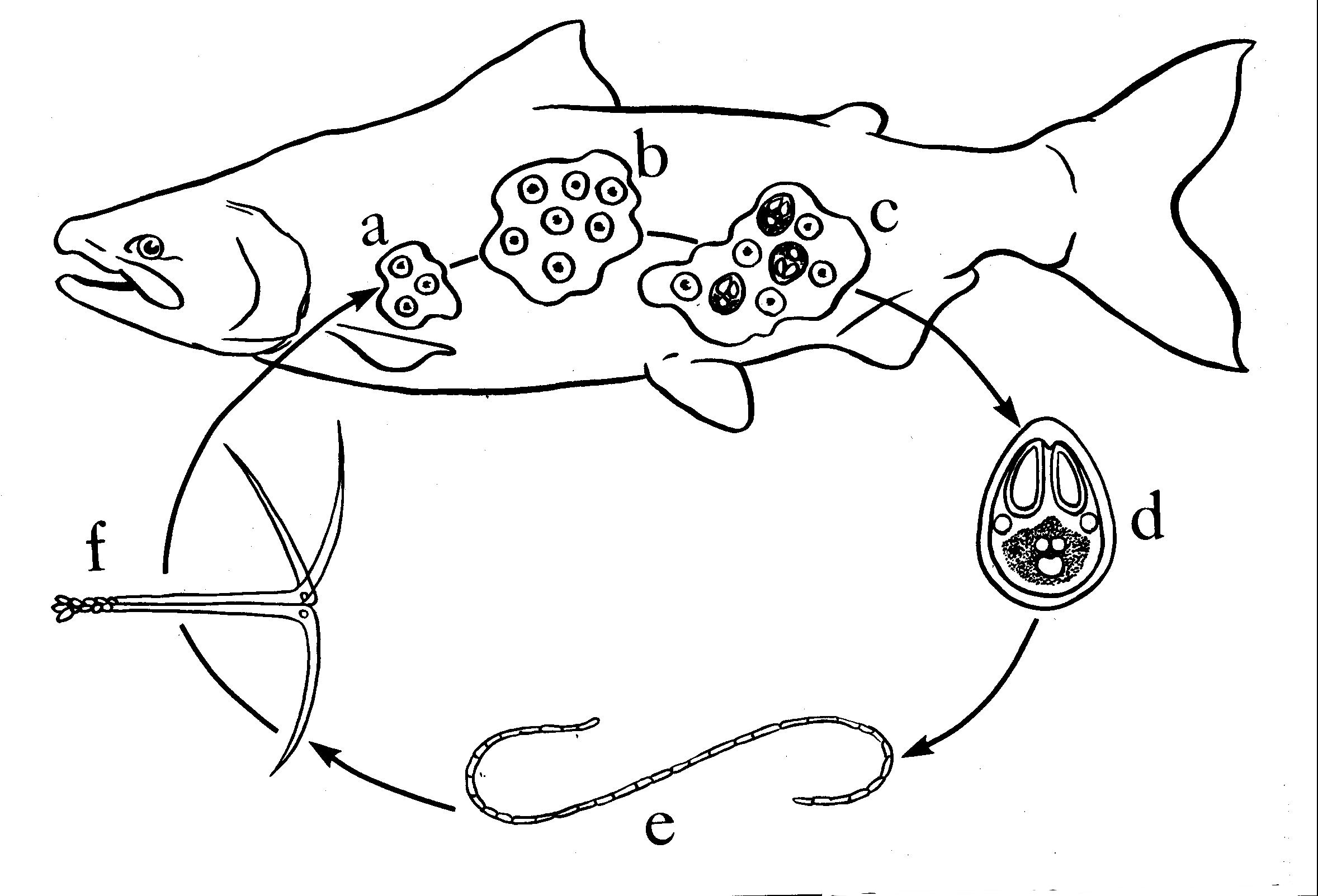 Click for high resolution image | Simplified life cycle and development of the Myxozoa, exemplified by Myxobolus sp. (from Kent and Poppe 1998). a and b vegetative development results in formation of a multinucleated plasmodium (trophozoite) with many daughter cells; c daughter cells develop into multicellular Myxospores; \\d myxospores are released from the fish to complete development and transmission of the parasite - spores are released after death for most histozoic species, whereas spores are released in feces or urine in coelozooic species; e annelid worms become infected after ingestion of myxospores from fish. f following complex development, the “actinospore” is released from the worm and infects the fish host to complete the life cycle. cell sarcomas. A. Zebrafish with capillary hemangioma behind eye. Tumor growth has caused prominent exophthalmia. |
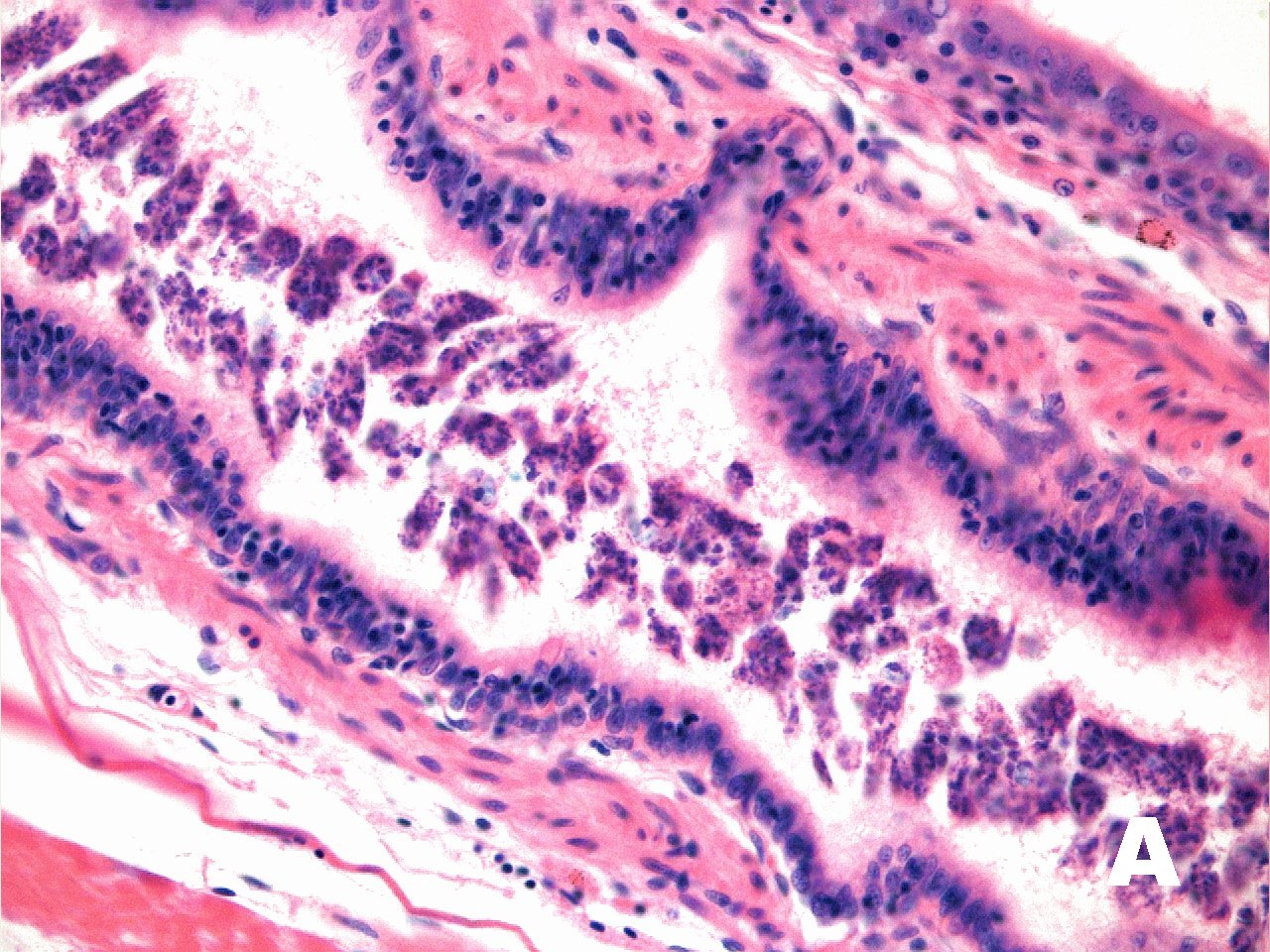 Click for high resolution image | A. Trophozoites in lumen of common mesonephric duct. H&E. |
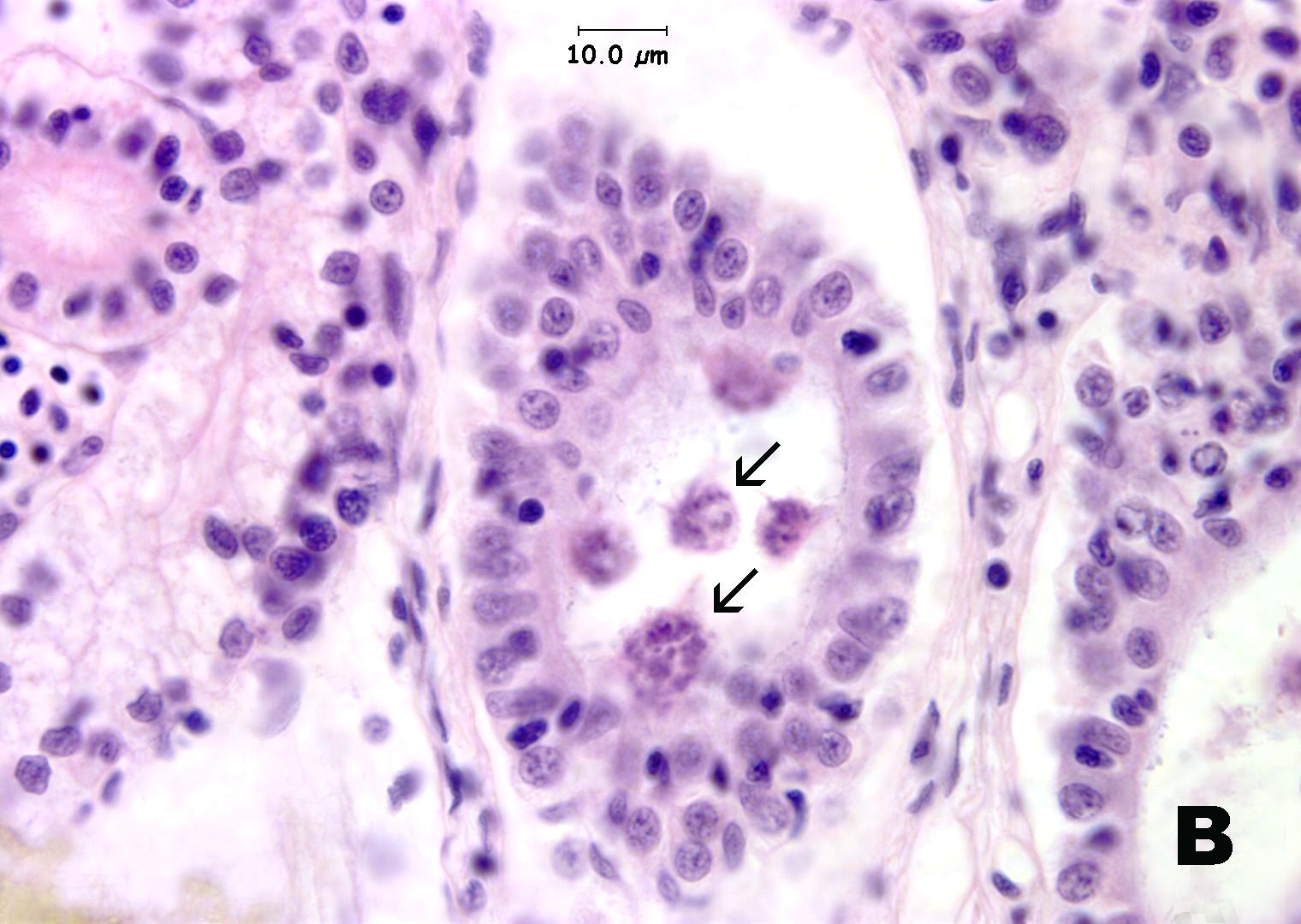 Click for high resolution image | B. Trophozoites in kidney tubules (arrow). H&E. |
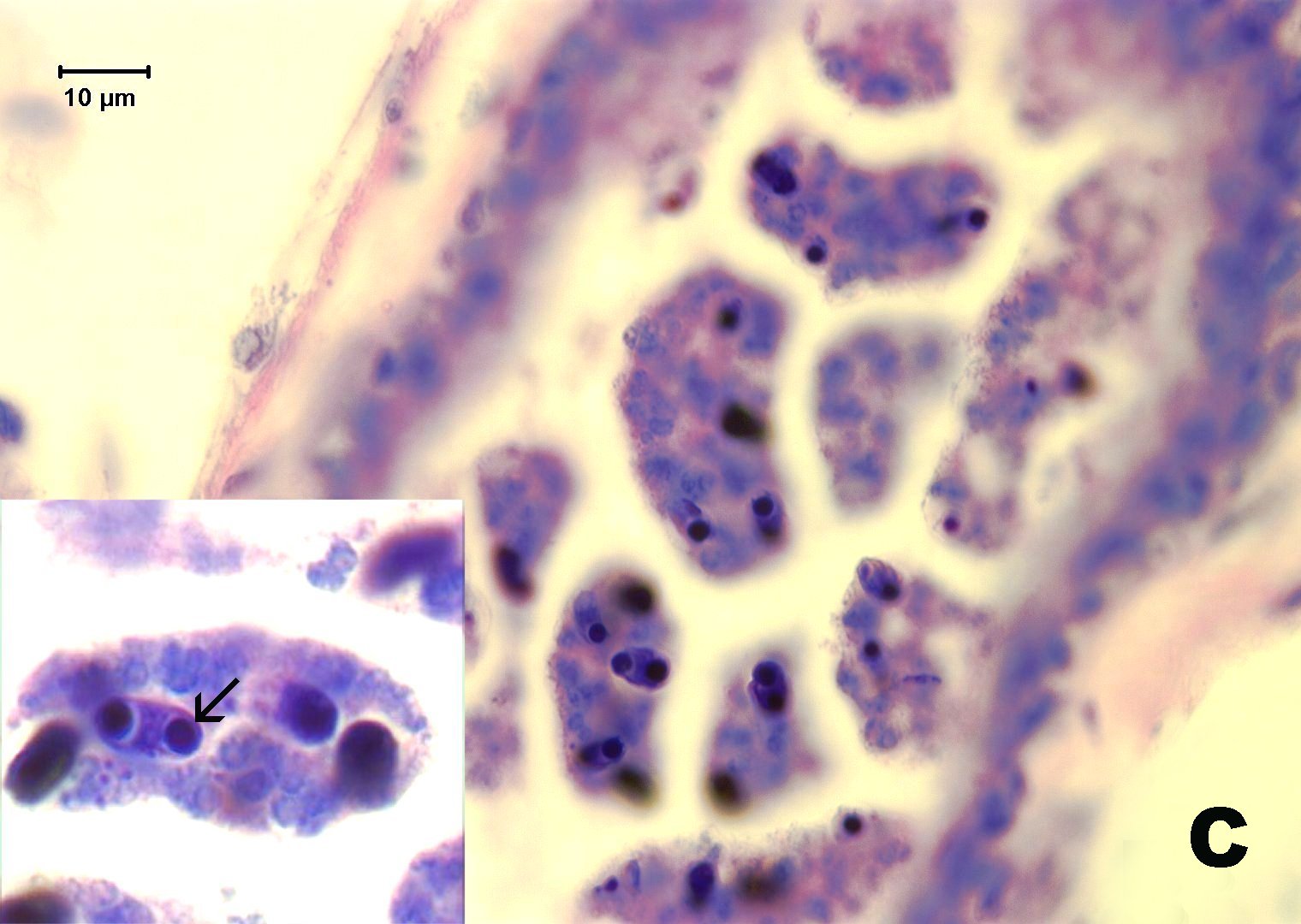 Click for high resolution image | C. Giemsa stain reveals myxospores with two polar capsules at opposite ends of spores. |
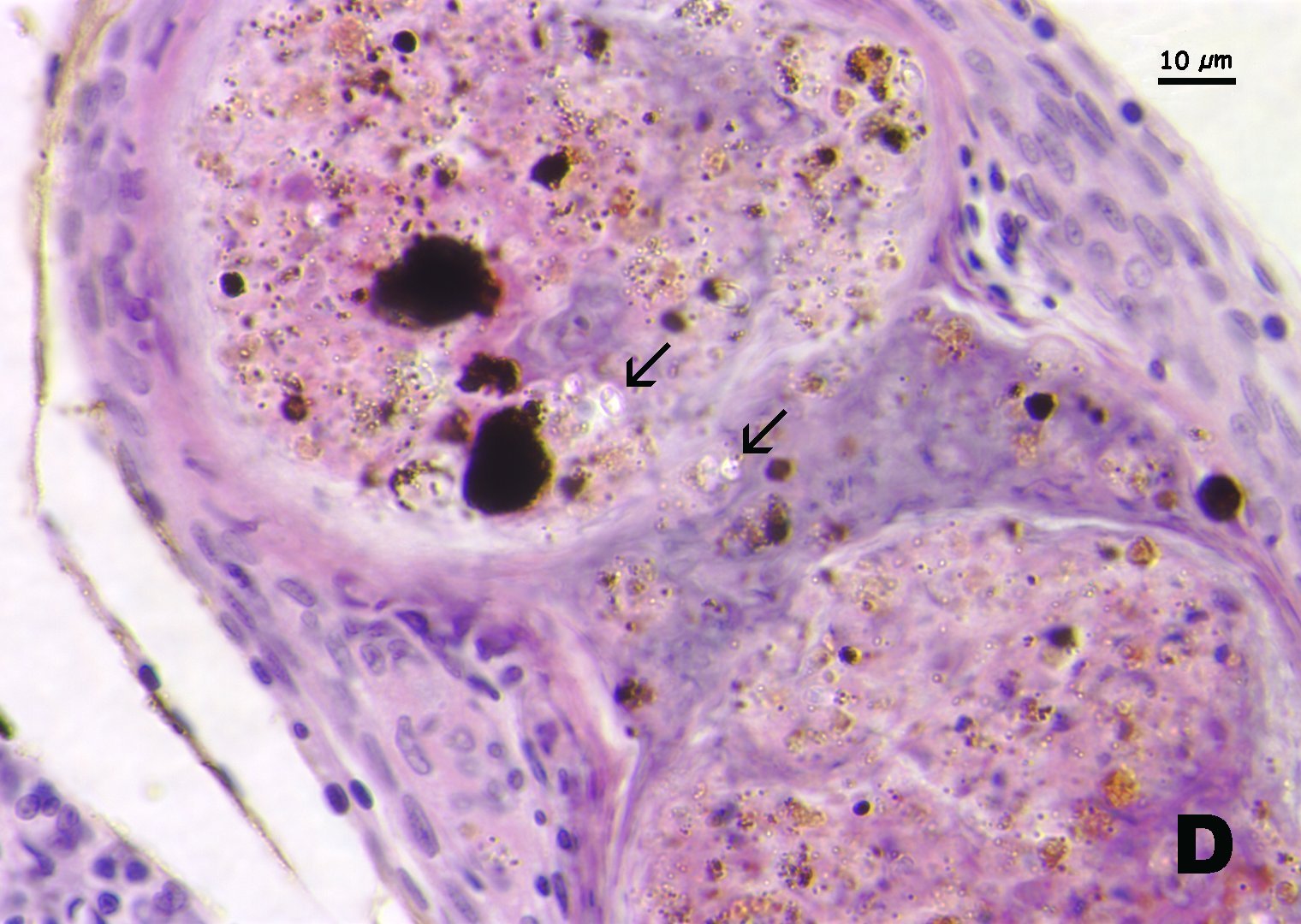 Click for high resolution image | D. Granulomas in kidney associated with myxospores (arrows). Note that spores appear clear with H&E stain. |