Table of Contents
Step-by-Step Cryopreservation: Pooling, Freezing, Thawing, and IVF Protocols

OVERVIEW
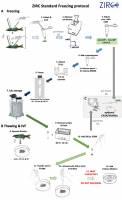
1. Collect sperm and pool in extender E400G
2. Measure cell density
3. Dilute with E400G. Target: 4×108 - 1.6×109cells/ml
4. Add RMMB Cryoprotectant solution (1V sperm + 3V RMMB)
5. Aliquot into cryovials (20 µl) and cap
6. Freeze in dry ice
7. Store in LN2
8. Squeeze eggs, pool clutches as needed; store max. 10 minutes in humid chamber
9. Retrieve & thaw sample
10. Add SS300
11. Transfer to eggs
12. Activate to fertilize
13. Do NOT swirl or mix fertilized eggs/dish. Wait 2 minutes
14. Add embryo medium; rear embryos
A. Collection and pooling of sperm
1. Prepare a 0.5 mL microcentrifuge tube with E400 for each fish line (see Note1) to estimate E400 starting volume). Use a separate microcentrifuge tube for each fish line.
To make 1 L of E400, combine the following in:
- 800 mL dH2O:
- 9.70 g KCl,
- 2.92 g NaCl,
- 2.0 mL of 1.0 M CaCl2 (or 0.29 g CaCl2 * 2H2O),
- 1.0 mL of 1.0 M MgSO4 (or 0.25 g MgSO4 * 7H2O),
- 1.8 g D-(+)-Glucose,
- 7.15 g HEPES.
- Add dry components first, stir to dissolve, add liquid components, and stir.
- Adjust the pH to 7.9 with 5 M KOH and bring the final volume to 1000 mL with dH2O.
It is advisable to check the osmolality of E400, which should be close to 400 mmol/kg. The solution should be filter sterilized and stored at 4 ºC. The resulting E400G contains 130 mM KCl, 50 mM NaCl, 2 mM CaCl2, 1 mM MgSO4, 10 mM D-(+)-Glucose, 30 mM HEPES-KOH (pH 7.9).
2. Keep E400 and the collected sperm on ice throughout the collection and prefreezing procedures.
3. Sedate or preanesthetize males as needed for at least 10 min before the procedure (see Note2)). Prepare and a tricaine (MS-222) stock solution as follows:
4 g/L in dH2O, pH adjusted to 7.0 with Tris-HCl (pH 9.0). Store in amber bottle at 4 ºC. To prepare the MS-222 preanesthesia solution of 48 mg/L, dilute 12 mL MS-222 stock solution in 1000 mL fish water.
4. For anesthesia, dilute 4.2 mL tricaine stock solution in 100 mL fish water (168 mg/L). Anesthetize two to three males in 168 mg/L MS-222.
5. Briefly rinse a male in phosphate buffered saline (PBS) isotonic fish rinse, dry it by rolling gently on a soft and absorbent paper towel (see Note3)), and place it belly up in a dampened sponge/foam holder (in a 35 x 10 mm Petri dish). The isotonic PBS fish rinse is prepared from PBS (pH 7.4) powder packets (Sigma #P3813) that are dissolved in 870 mL dH2O. The final osmolality should be approximately 315 - 325 mmol/kg. Place the sponge holder under a dissecting microscope with incident lighting.
6. Place the end of a calibrated 10 mL borosilicate glass microcapillary (Drummond #2-000-010) on the urogenital opening.
7. Use rubber-tipped Millipore forceps (Millipore #XX6200006P; rubber tips made from heat-shrink tubing) to apply gentle abdominal pressure to the sides of the male. Move the forceps gently from anterior to posterior toward the urogenital opening. Collect sperm into the microcapillary.
8. Expel sperm immediately into the E400 solution in the microcentrifuge collection tube.
9. Transfer fish into fresh system water for recovery from anesthesia.
10. Continue collecting sperm from all males from the same family/stock and pool into the same E400 microcentrifuge tube. Repeat steps 4 - 8 for each male.
B. Estimation of sperm cell densities and sample dilution
We use the ZIRC NanoDrop 2000 Calibration Curve and Sperm Density Calculator to determine the cell density by light absorption at 400 nm (see Note4)). The worksheet we use to determine NanoDrop cell counts is available in the Cryopreservation and IVF section on the ZIRC site: https://zebrafish.org/wiki/protocols/cryo (see Note5)).
1. Estimate the volume of pooled sperm in E400 in the microcentrifuge tube for each line using a Pipetman. Draw the sperm into the tip and adjust the pipette volume until all of the solution fills the tip. Record the estimated volume for later use. While measuring, gently pipette the sperm to mix completely.
2. Prepare a 1:10 dilution of the sperm solution in E400 for a density measurement. If the collected sample appears less opaque (i.e., less concentrated), a 1:5 dilution can be used. Pipette 9 µL (or 4 µL for a 1:5 dilution) E400G into a 0.6 mL microcentrifuge tube and add 1 µL of the sperm suspension.
3. Mix the diluted sample by flicking the tube and keep the tube at room temperature. Optional: Use separate (colored) microcentrifuge tubes for each line (see Note6)).
4. Calibrate A400 absorption of a blank E400G sample using the NanoDrop’s Cell Cultures menu. Set the Absorbance Cursor to 400 nm and add 1.5 µL E400 to the spectrophotometer. Read the E400 blank sample to verify that it has set the instrument’s readings close to 0. If a blank sample reads higher than 0.004, repeat the blanking procedure.
5. To measure the absorption of a sample, enter the sample ID on the Measure Cell Cultures page.
6. Mix the sample well by flicking or using a vortex mixer set at intermediate speed (~1300 rpm).
7. Immediately load 1.5 µL of the diluted sperm and read the cursor absorbance (AOD400; select the measure button in the left-hand corner). Readings above 0.2 are acceptable, and you can proceed with the dilution. Any reading at or below 0.2 should be diluted 1:5 and repeated.
8. Measure each sample three to five times and calculate the average A400 for all samples. A minimum of three successful readings is optimal.
9. Clean the NanoDrop using a clean Kimwipe and deionized water. Wipe the top arm (mirror) and the bottom lens with a moistened Kimwipe and then dry completely with a Kimwipe before closing the arm.
10. Calculate the cell density for each sample with the averaged A400 using the ZIRC NanoDrop 2000 Calibration Curve and Sperm Density Calculator or similar lab specific calibration curve.
11. Dilute the sperm with E400 according to the desired number of samples or sperm concentration. Optimal sperm cell densities should be between 4.0*108 and 1.6*109 cells/mL. This density range will result in samples with 2.0*106 - 8.0*106 cells/sample (see Note7)).
C. Sperm freezing
1. Prepare RMMB cryoprotective medium (100 mL) in the following order:
- Combine 20.0 g D-(+)-raffinose pentahydrate (Sigma R7630 or 83400) and 70 mL dH2O in a 250 mL beaker.
- Then, place the beaker in an evaporating dish (Pyrex 3140) or large beaker containing hot water (~70 ºC) on a stir plate.
- Stir the mixture until the raffinose is completely dissolved.
- Add 2.5 g skim milk (Difco #232100) and stir until it is completely dissolved.
- Let the solution cool to room temperature and add 3 mL of 1 M Bicine-NaOH (8.0).
- Add 6.67 mL absolute methanol (acetone-free, absolute, Certified ACS Reagent Grade), transfer the mixture to a 100 mL volumetric flask, adjust the final volume to 100 mL with dH2O, and mix by inversion three to four times.
- Transfer the solution to two 50 mL conical tubes and centrifuge at 15,000g for 20 min at 25 ºC.
- Transfer the clear supernatant into a clean beaker and aliquot into 1.5 mL microfuge tubes, 1 mL each, or a different convenient volume for daily use.
- Store the RMMB solution frozen at -20 or -80 ºC until use.
The resulting RMMB cryoprotective solution will contain 20 % (w/v) D-(+)-Raffinose pentahydrate, 2.5 % (w/v), Difco skim Milk, 6.67 % (v/v), Methanol, and 30 mM Bicine-NaOH.
2. Fill a Styrofoam container or cooler (9 inch or 23 cm min. depth) with powdered dry ice made from liquid CO2 (ZIRC E400_RMMB Sperm Cryopreservation & IVF Protocol https://zebrafish.org/wiki/protocols/cryo ) (see Note8)).
3. Prepare 15 mL Falcon tubes (Falcon 352,096) with an empty Matrix cryovial tube (0.5 mL Matrix Screw Top Storage Tubes, Thermo Scientific, Item #3745-BR or 2 mL Corning vials, Item #430488) functioning as a spacer in each along with the Falcon tube caps so that the tubes are ready to hold samples immediately after the cryovials have been capped.
4. Prepare labeled sample cryovials as needed prior to freezing (all samples should be labeled with the freeze date and allele number and/or line identification number). Additional vial color coders (colored caps or cap inserts specific for vial type) are helpful to distinguish freeze events or different stocks.
5. If you are using a multicapper, prepare it with the appropriate caps already attached before adding cryoprotectant (RMMB) to sperm.
6. For each sample, determine the volume of RMMB (RMMB volume = 3 x the sperm volume, see Note9)).
7. Add RMMB to sperm and mix by pipetting.
8. Immediately aliquot 20 µL into the prelabeled 0.5 mL cryovials.
9. Without delay, cap the cryovials (use an automated capper for rows of eight tubes, if available) and place the cryovials into the 15 mL conical tubes (containing a Matrix cryovial spacer).
10. Cap the conical tubes and drive the tubes into the dry ice until the caps are flush with the surface (see Note10)).
11. Freeze the samples in dry ice for 20 - 45 min and then quickly transfer them to a cryogenic freezer box submerged in LN2.
D. Egg collection
1. Place females in a tank with preanesthesia solution at least 10 min before full anesthesia. However, fish can be held in a preanesthesia solution for several hours (see Note11)).
2. Anesthetize females in tricaine/MS-222 solution.
3. Rinse fish in isotonic PBS and blot dry by gently rolling on a paper towel.
4. Place fish on its side in a small Petri dish (35 or 60 mm).
5. Dampen fingers with PBS fish rinse.
6. Expel eggs by applying gentle (light) finger pressure on the ventral abdomen and move your finger from anterior to posterior. Eggs will be expelled readily if the female is ready (see Note12)).
7. Transfer the female to a recovery tank.
8. Combine several clutches of eggs if needed by gently moving eggs to another dish with a fine-tipped paint brush dampened with isotonic PBS (see Note13)).
9. Maintain pooled eggs in a closed dish in a moisture chamber at room temperature no longer than 5-10 min before IVF.
E. Sperm sample thawing
1. To prepare 1L Sperm Solution SS300,
- combine 8.2 g NaCl,
- 5 mL of 1 M KCl (or 0.37 g KCL),
- 1 mL of 1 M CaCl2 (or 0.15 g CaCl2*2H2O),
- 1 mL of 1 M MgSO4 (or 0.25 g MgSO4*7H2O),
- 1.8 g D-(+)-Glucose,
- 20 mL of 1 M Tris-Cl (pH 8.0)
- in 800 mL dH2O.
- Add all dry ingredients first and stir until they are dissolved. Then add liquid ingredients and mix.
- Bring the final volume to 1000 mL with dH2O and check the osmolality (should be approximately 300 mmol/kg).
- Filter sterilize the solution and store it at 4 ºC.
The resulting SS300 solution will contain 140 mM NaCl, 5 mM KCl, 1 mM CaCl22, 1 mM MgSO4, 10 mM D-(þ)-Glucose, and 20 mM Tris-Cl (8.0). It is used when the cryopreotective medium already contained skim milk. The milk helps to prevent sperm tails from sticking and tangling and is thought to contain antioxidants that protect against oxidative damage during cryopreservation and thawing.
2. To prepare Sperm Solution SS300 with 2 mg/mL Difco Skim Milk (SS300 + milk),
- Add 100 mg Difco Skim Milk to 50 mL SS300 and stir or vortex to dissolve.
- Aliquot the solution into microcentrifuge tubes and store frozen at -20 ºC.
- Thaw and use at room temperature.
3. Remove the cryovial from the LN2 and quickly open the cap to vent any LN2 in the vial (see Note14)).
4. Thaw the cryovial in a 38 ºC water bath until the frozen pellet is less than 3 mm in diameter (takes approximately 10 - 15 s).
5. Immediately add 200 µL room-temperature SS300 solution to the cryovial. If you are thawing sperm that was frozen without milk (see Note15)), add 2 mg/mL Difco Skim Milk (Difco #232100) to the SS300 solution. See Note16) for an optional postthaw motility assessment.
F. In vitro fertilization
1. Gently mix the sperm 1 - 2 times with a micropipette and transfer the sample to the eggs:
- Slide the pipette tip sideways along the bottom of the Petri dish from the edge of the pile of eggs into the center.
- Expel the sperm into the mass of eggs (not on top of the eggs).
2. Add 320 µL dH2O to the eggs to activate the sperm.
3. Start a 2-minute countdown timer.
4. Do not move, mix, or swirl the dish; let it sit completely undisturbed for 2 minutes, then flood the dish with embryo medium.
5. Determine the fertilization rate 2 - 4 h postfertilization or as soon as cell divisions are recognizable.
- Count embryos and remove unfertilized eggs (see Note17)).
G. Optional sperm motility assessment
A good way to assess the quality of sperm is to observe its motility. Computer-assisted sperm analysis software systems provide an objective and comprehensive quantification of the density and motility parameters, but a manual, subjective assessment is feasible in most labs and is sufficient for most sperm-freezing applications. A compound microscope with a 10x or 20x objective and DIC or dark field illumination is ideal. Osmolality affects both the speed and duration of sperm motility. Fresh (prefreeze) sperm will be faster and have a higher percentage of motile cells than postthaw samples.
To estimate the prefreeze motility,
1. Place 6 µL dH2O on a microscope slide.
2. Add 0.5 - 1 µL of the final sperm dilution (in E400G) to the drop and then mix the drop and spread it quickly with the pipette tip.
3. Observe immediately (see Note18)).
To estimate the postthaw sperm motility,
1. Thaw a sperm sample in a water bath as described below,
2. add 200 µL of SS300 solution to the thawed sperm, and mix gently.
3. Remove 10 - 20 µL of the sample for motility assessment (see Note19)).
4. Place 5.8 µL dH2O on a slide and add 4 µL of thawed sperm/SS300 solution (see Note20)).
5. Mix briefly with the pipette tip on the slide and observe immediately.
Protocol Notes
- Short-fin fish: (# males-2) x 5 µl E400G
and
- Long-fin fish: (# males-4) x 5 µl E400G
(1) further dilution of sperm (if possible) generates more samples and a longer lasting resource, and
(2) an optimal ratio of cells to available salt and buffer molecules ensures that the solution toxicity and osmotic shock are reduced, and as a result, cells suffer less damage during freezing and thawing.