Table of Contents
General Diagnostics & Necropsy Protocols
The small size of zebrafish present some unique challenges, and at the same time advantages for conducting necropsies and other diagnostic tests. Our knowledge of the diseases of zebrafish is relatively limited compared to those of food fishes reared in aquaculture. Until recently, most diseases of zebrafish that have been documented are those that are common problems in ornamental freshwater fishes (e.g., Piscinoodinium). With other fish species, it was not until they were recognized as commercially important and reared in captivity in large numbers did specific diseases become recognized.
Viral diseases are often devastating in aquaculture enterprises. However, as many viruses are very host specific, they are usually first identified only when a fish species is reared in large numbers in captivity. Identification of “new” viral diseases in fishes often requires establishment of new cell lines from the host under investigation. At present, viruses are not recognized as causes of disease in zebrafish, and thus zebrafish are not routinely examined for viruses. However, zebrafish cell lines are available.
Likewise, with bacterial infections, aside from mycobacteriosis, only common, opportunistic Gram negative bacteria such as Aeromonas hydrophila and surface infections by gliding bacteria are occasionally encountered. Given the above, our primary approach for diagnostics has been histopathology. This allows for interpretation of pathological changes at the tissue and cell level. Moreover, the small size of zebrafish allows for preparation of the entire fish on one slide.
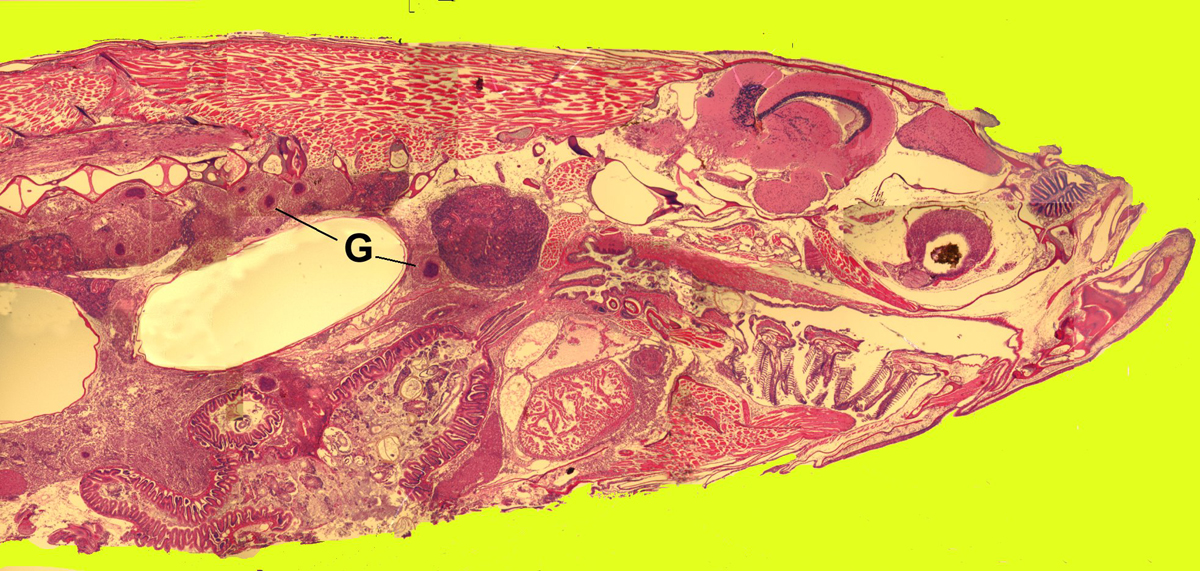 Click for high resolution image | Sagittal section of whole zebrafish with mycobacteriosis G = granulomas in the kidney and viscera due to the bacterial infection |
The mere presence of a pathogen in a sick fish does not necessarily mean that it is the cause of the disease. It is important, therefore, to conduct a thorough investigation, beyond just detecting the presence or absence of known pathogens, to determine the cause of the disease. Histological examinations, in addition to gross necropsies and in vitro culture of microorganisms, are often required to determine the association of the observed pathogens in the disease being investigated.
Diagnosis of a disease begins at the research facility. Some of the most crucial data are collected by the staff maintaining the fish by thorough record keeping and careful observations of affected fish. The following information is important for disease diagnosis and implementing control strategies. Astrofsky et al. (2002) recently published a guide for conducting clinical investigations on laboratory zebrafish.
History
To assist with obtaining an accurate diagnosis, the following information should be obtained: origin (stock and strain), previous disease problems in the affected population, and husbandry conditions (e.g., water hardness, diet, medication history, system design, and mortality rate).
Behavior
Fish behavior may be useful for indication of an emerging disease and some behavioral changes are useful for presumptive diagnoses. For example, if fish are flashing (rubbing on the sides of tanks), this may indicate that they are infected with external parasites. Other indications of disease include cessation of feeding, lethargy, and abnormal position in the aquarium (e.g., at surface or at the bottom). Abnormal respiratory pattern may indicate gill damage, and whirling or spiraling swimming often indicates neurological damage.
Selecting Fish for Necropsy
Examinations should be conducted on diseased fish that are collected while still alive (moribund). It is important to determine the primary cause of mortality and to differentiate this from secondary or opportunistic pathogens that may have taken advantage of already diseased fish. To accomplish this, several affected fish should be examined whenever possible. It is also useful to include apparently normal, asymptomatic fish so that early pathological changes and the underlying cause of morbidity can be determined. Dead fish may be suitable for some parasitological examinations and for observing obvious macroscopic pathological changes. However, bacteriological examinations conducted on dead fish can yield misleading results, and many histological changes are obliterated by post-mortem autolysis.
External Examination
The small size of zebrafish makes conducting a necropsy somewhat tedious, but with patience and practice this can be efficiently accomplished with the aid of a stereo dissecting microscope. Note surface abnormalities (e.g., frayed fins, cloudy eyes, ulcers, skin discolorations, parasites, and tumors). Prepare wet mounts of the skin mucus and a few scales by scraping the surface of the fish with a coverslip and placing the coverslip on a glass slide. Some water may be added to the preparation so that the area between the slide and the coverslip is completely filled with liquid. Examine the wet mount with a compound microscope, starting with low power. Reducing the light and lowering the condenser will produce higher contrast, which will make microscopic parasites and other pathogens more visible.
Gills
Remove the operculum. Note color of gills (pale gills usually indicate anemia). Check for parasites, cysts, excessive mucus, and hemorrhages with a dissecting microscope. Prepare a wet mount by removing a few filaments with scissors, placing the filaments in a large drop of freshwater on a glass slide, and overlaying with a coverslip. Examine for small parasites, fungi, and bacteria using a compound microscope.
Internal Examination
Open the visceral cavity. Note if ascites, hemorrhages or other abnormalities are present. Expose the kidney by removing the swim bladder and note any kidney abnormalities. Many diseases cause enlargement or discoloration of the kidney, but this may be difficult to see in zebrafish due to their small size. Examine the heart for any abnormalities. Multiple, whitish cysts in the visceral organs is suggestive of Mycobacterium infections. Examine squash preparations of organs with a compound microscope to detect encysted parasites, fungi or granulomas. Squash preparations are made by removing a small piece of tissue, and gently squashing it between a slide and coverslip so that a thin preparation suitable for examination with a compound microscope or dissecting microscope is made.
Imprints
Leishman's Giemsa or Diff-Quik (Dade Behring AG, Newark, DE) stained imprints of kidney, or other affected organs are useful for detection of protozoa and bacteria. Remove a piece of tissue, blot on clean paper towel to remove most of the blood, and lightly touch the cut surface of the tissue on a clean glass slide. Several imprints from the same piece of tissue can be made on one slide. Air dry the preparation for approximately 1/2 h. Fix the slide for 5-10 min in absolute methanol for Giemsa stains or the fixative provided with the Dif-Quik kit. The slide can then be stained with Giemsa or Diff-Quik, or shipped to a diagnostic laboratory. For Mycobacterium, imprints and tissue smears are stained with acid fast stains.
Histology
It is critical to fix fish for histology as soon as possible after fish are killed to avoid post-mortem changes. If possible, do not use dead fish because significant autolytic changes may occur in 15-20 min after death. Fish are preserved in formaldehyde based fixative such as Davidson’s or Dietrich’s solutions for histology. Open the visceral cavity of the fish by cutting and removing a small section of the abdominal wall. Then place fish whole in the fixative at approximately 1:20 (v/v) tissue to fixative. We have found Dietrich’s or Davidson's solution to be the best all around fixative for processing whole zebrafish for histological examination. The entire fish can be processed (usually cut in sagittal sections) and representative organs can be visualized in multiple sections on one or two slides.
Electron Microscopy
Under special circumstances, electron microscopical examinations may be warranted. The general principles for histology applies for collecting specimens for electron microscopy, but freshness of tissues at fixation and proper infiltration of tissues is even more critical. Therefore, small pieces of tissue should be minced in cold glutaraldehyde based fixative into pieces about 2 mm3 maximum. The fixed tissue is stored overnight in this solution, then transferred to the appropriate buffer solution. Samples in EM fixatives and buffers should be refrigerated. There are many EM fixatives and buffers. The Appendix provides a recipes for Millonig’s buffer and fixative. Transmission electron microscopy can be performed with limited success on tissues fixed in neutral buffered formalin, but is very poor with acidic fixatives, such as Davidson's or Dietrich’s solutions.
Bacteriology
Some bacterial infections, such as surface gliding bacteria and Mycobacterium spp., can be identified by simple Gram or acid fast stains (e.g., Ziehl Neelsen). However, the diagnosis of other bacterial diseases requires isolation of the bacteria in culture. In this case, live fish should be delivered to the laboratory, but this may not be practical in some situations. For this reason, methods for obtaining initial cultures are provided below. The bacterial cultures can then be sent to a laboratory for complete identification.
Use only freshly sacrificed fish for bacteriological examinations as dead fish from the tanks are essentially worthless. Disinfect the surface of the fish with 70 % ethanol. Flame-sterilize the dissecting instruments. Two methods are used for obtaining inocula from the kidney. For the visceral method, open the visceral cavity by making an incision in abdominal wall. Make sure not to cut into the gastrointestinal tract. Then push aside the swim bladder with flame-sterilized forceps and insert a sterile swab or loop into the kidney. For the dorsal method, enter the kidney by lateral incision dorsal to the kidney (i.e., near the lateral line). Streak the specimen on Tryptic Soy Agar or blood agar bacteriological plates, seal the plates, keep at a room temperature, and send the plates to the microbiology laboratory for further diagnosis.
For gliding bacteria (Cytophaga, Flexibacter and Flavobacterium spp.), we recommend Cytophaga Medium. Although the lesions may exhibit massive numbers of gliding bacteria, other bacteria (e.g., Aeromonas spp.) may overgrow the former. Therefore, the best way to obtain pure cultures of gliding bacteria is to homogenize the infected tissue (e.g., gills, skin and muscle) in sterile water, and inoculate plates in serial log dilutions.
Gram-stained preparations may reveal bacteria when they are numerous in infected tissue. Smear or imprint suspect tissues thinly on a glass slide, air dry and fix the slide by gently heating the slide over an open flame for 3-5 seconds. Gram stain kits are available from scientific supply houses and include instructions for their use.
Virology
As with bacterial diseases, isolation of viruses in culture may be required to diagnose a viral disease, and culture is best conducted on tissues collected from freshly killed fish. If this is not practical, the fish should be refrigerated for no longer than 24 h before examination. As a last resort, fish for virus examination can be frozen. The specimens are then transported to a qualified fish virology laboratory. At present, there are no known viral diseases of zebrafish colonies. However, as has been the case with other relatively new forms of fish culture, as fish health researchers thoroughly investigate outbreaks and incorporate tissue culture in their examinations, it is very likely that “new” viral diseases will be recognized in zebrafish.
Molecular Biology
DNA-based diagnostic tests utilizing polymerase chain reaction (PCR) are becoming increasingly common in fish health diagnostics because they are very sensitive and specific. With zebrafish, we have developed a PCR test for the neural microsporidian Pseudoloma neurophilia, and Astrofsky et al. (2000) used PCR tests to identify and differentiate different species of Mycobacterium. Because of the extreme sensitivity with PCR, great care should be taken to avoid cross contamination between samples. Bleach instruments between samples. Samples are either frozen immediately or preserved in ethanol. Mixed results are achieved when using formalin preserved tissues for genotypic analysis. Moore et al. (2001) found that decalcification procedures, which are routinely used when processing zebrafish for histology, are particularly detrimental for molecular analyses.
Transport of Specimens
It is important that you contact the diagnostic laboratory prior to shipping live fish. If possible, food should be withheld from fish for 24 hours prior to packaging and shipping. The water used for shipping should be held separate from the fish before packaging. Sodium chloride can be added to the shipping water at a rate of 2 ppt (2.0 g NaCl per liter water) to help reduce osmotic stress. Fill shipping bags 1/3 full of clean water and gently place fish into the bag. The bag should be sealed such that an approximate ratio of 1/3 water, 2/3 air is maintained. Bags are typically sealed by twisting the top and then folding the twisted end over and securing it onto itself with a rubber band. The bag is then placed within a second bag and sealed in the same manner. Place the bag in an insulated shipping container. Live specimens should be shipped early in the week to ensure arrival and adequate processing time.
It is not always possible to obtain live or fresh specimens. If only dead fish can be provided for laboratory examination, they should be refrigerated and examined within 24 h. If fish cannot be delivered to a laboratory within 24 h, then fish should be preserved by fixation in tissue preservatives for histology unless otherwise indicated. Each method of preservation has certain advantages and disadvantages, as indicated in following table.
Transport of Tissues in Fixative. For shipment of preserved fish, replace fixative with 70% alcohol and soak overnight. Drain off excessive fluids, wrap tissues in alcohol-soaked paper towels, and seal in a leak-proof container.
Transport of Frozen Fish. Freeze fish in a plastic bag and ship in an insulated container with ice packs.
Transport of Refrigerated Fish. Place fish in a plastic bag and surround the bag with ice or ice packs. Ship in a leak-proof, insulated container.